A Note on RSV Scenario Modeling Hub Round 1 2024-2025 (December 17, 2024)
The RSV Scenario Modeling Hub has generated early season hospitalization estimates for the 2024-25 season over a 45-week period running from July 28, 2024 to June 7, 2025. Four intervention scenarios were considered, representing the impact of maternal vaccines, long-acting monoclonal antibodies for infants (nirsevimab), and senior vaccines, in conjunction with a counterfactual scenario where these new interventions were not implemented. Intervention scenarios assumed different timings of infant interventions (classic timing following current recommendations, with a campaign starting in September for maternal vaccines and in October for nirsevimab, compared to early timing where immunizations start 1.5 months earlier), combined with optimistic and pessimistic assumptions about waning of vaccine-induced immunity in seniors aged 60 years and older. Intervention coverage and effectiveness were the same for all scenarios and were based on recent real-world data and projections of uptake. Ensemble projections are based on contributions from 12 teams using the trimmed linear opinion pool aggregation approach. All-age and age-specific estimates of RSV hospitalizations are provided nationally and for the 12 states that contribute to multi-year RSV-NET surveillance.
Our main findings include:
- On a national scale, and compared to the counterfactual, we project that 17% (95% Confidence Interval [CI]: 15-18%) of seasonal RSV hospitalizations, or 29,300 (95% CI: 23,200–35,500) hospitalizations, will be averted in the scenario with slow vaccine waning in seniors and early timing of infant interventions (scenario A), compared to non-intervention scenario (scenario E). Intervention benefits are projected to be highest in the targeted age groups, with hospitalization reduction of 31% (95% CI: 24-38%) among infants and 22% (95% CI: 19-25%) among seniors for scenario A vs E.
- These results correspond to 0.56 (95% CI: 0.42-0.71) infant hospitalizations averted per 100 infants receiving immunization during the 2024-25 season (i.e., via maternal vaccines and nirsevimab) in the most optimistic scenario (scenario A). For seniors, it corresponds to 0.07 (95% CI: 0.05-0.09) senior hospitalization averted per 100 senior vaccine doses administered across the 2023-2024 and 2024-2025 seasons.
- Assumptions about vaccine waning among seniors affect projected intervention benefits. In scenarios assuming a 50% decay in vaccine protection in the second year after vaccine receipt (i.e., VE declines from 75% in the first year to 38% in the second year, scenario B), we project that 9,500 (95% CI: 5,000-14,700) senior hospitalizations will be averted. This compares to 13,000 (95% CI: 9,100-16,800) hospitalizations averted if the vaccine maintains robust protection for two years (i.e., VE decays by only 10%, from 75% to 68%, scenario A). Further, there is a statistically significantly greater reduction in senior hospitalizations if the vaccine maintains robust protection over two years, with 7% (95% CI: 4-10%) more hospitalizations averted by scenario A (slow vaccine waning) than scenario B (fast waning).
- The timing of infant interventions has little impact on intervention benefits. For instance, we project 14,300 (95% PI: 10,600-18,100) infant hospitalizations averted if the immunization campaign starts early, compared to 13,700 (95% PI: 9,900-17,600) if the timing is as currently recommended. The number of hospitalizations averted does not significantly differ based on the timing of infant interventions, with the percent reduction in infant hospitalizations between scenarios A and C being 0% (95% CI: -6-5%).
- The peak and cumulative hospitalization burden of the 2024-25 RSV season is likely to remain lower than that of last season and this is consistent across all scenarios. On a national scale, RSV activity is most likely to peak from mid-December to mid-February, with the earliest peak expected in Southeastern States (Georgia, Tennessee) and the latest peak in Western states (Utah, Colorado, New Mexico).
- The combined hospitalization impact of RSV, influenza, and COVID-19 is likely to remain below that of last season based on the 50% projection interval (SMH COVID-19 Round 18, scenario of high immune escape and boosters for all; combined with SMH Influenza Round 1 for 2024-25, scenario of usual vaccine coverage and dominance of A/H1 or A/H3; and combined with SMH RSV Round 1 for 2024-25, scenario of optimistic senior waning and classic timing of infant interventions). RSV is expected to represent around 13-15% of respiratory admissions throughout the winter.
A few caveats are worth noting:
- We did not find a clear benefit of an early immunization campaign among infants, likely because there is a trade-off between immunization early enough in the season to precede the peak of RSV activity and waning of immunization protection within the season. We note that this trade-off may eventuate differently in Florida, which has particularly early RSV timing but is not included in this analysis since it is not part of RSV-NET. Further, both infant scenarios assume a rapid uptake of catch-up doses of nirsevimab, within one month of the assumed start of the RSV campaign. If in practice catch-up immunizations are delayed due to supply shortages or other issues, the trade-off between the two immunization options may also ensue differently.
- Most models project that RSV timing will broadly return to normal in 2024-25 after several disrupted seasons due to COVID-19 interventions. Yet some aspects of the rebound remain poorly understood. Further, testing practices continue to evolve in the wake of the COVID-19 pandemic (e.g., increased use of multi-pathogen testing), which may affect reported hospitalizations in the RSV-NET system. Testing changes are not fully understood and not accounted for in the models.
- There is limited availability of calibration data from the RSV-NET hospitalization dataset, which covers only a fraction of 12 states (9% of the US population overall). Future work could focus on expanding these projections to all states as more data become available via the NHSN reporting system.
- Most models assume that RSV interventions do not affect susceptibility to infection or transmission, so that ensemble estimates of indirect benefits are minimal (i.e., hospitalization reduction in non-intervened individuals 1-59 years are within 0-3% depending on age group and scenario considered).
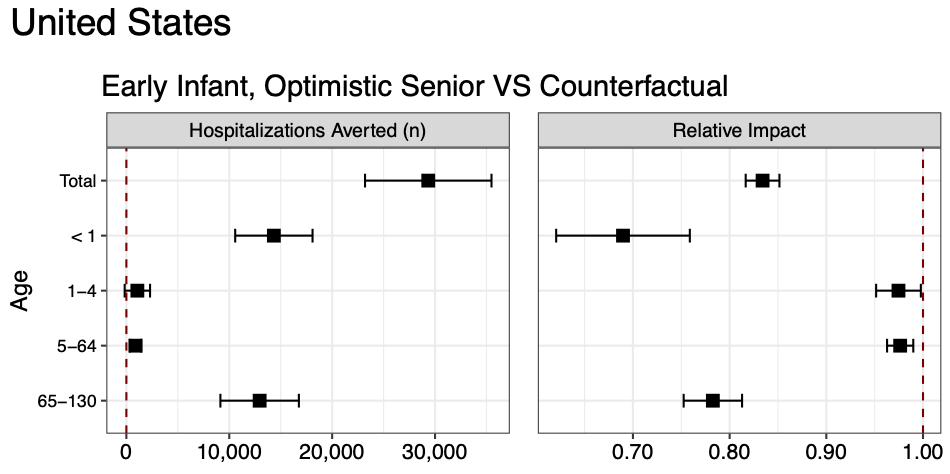
Figure 1. Hospitalizations averted by the most optimistic RSV intervention scenario, as compared to without new RSV vaccines and monocolonal antibodies, across the United States during the 2024-25 season. Infant nirsevimab monoclonals and maternal vaccination are expected to avert 14,300 (95% PI: 10,600-18,100) infant hospitalizations in the early scenario as compared to the status quo, and senior vaccination is expected to avert 13,000 (95% CI: 9,100-16,800) senior hospitalizations in the optimistic waning scenario
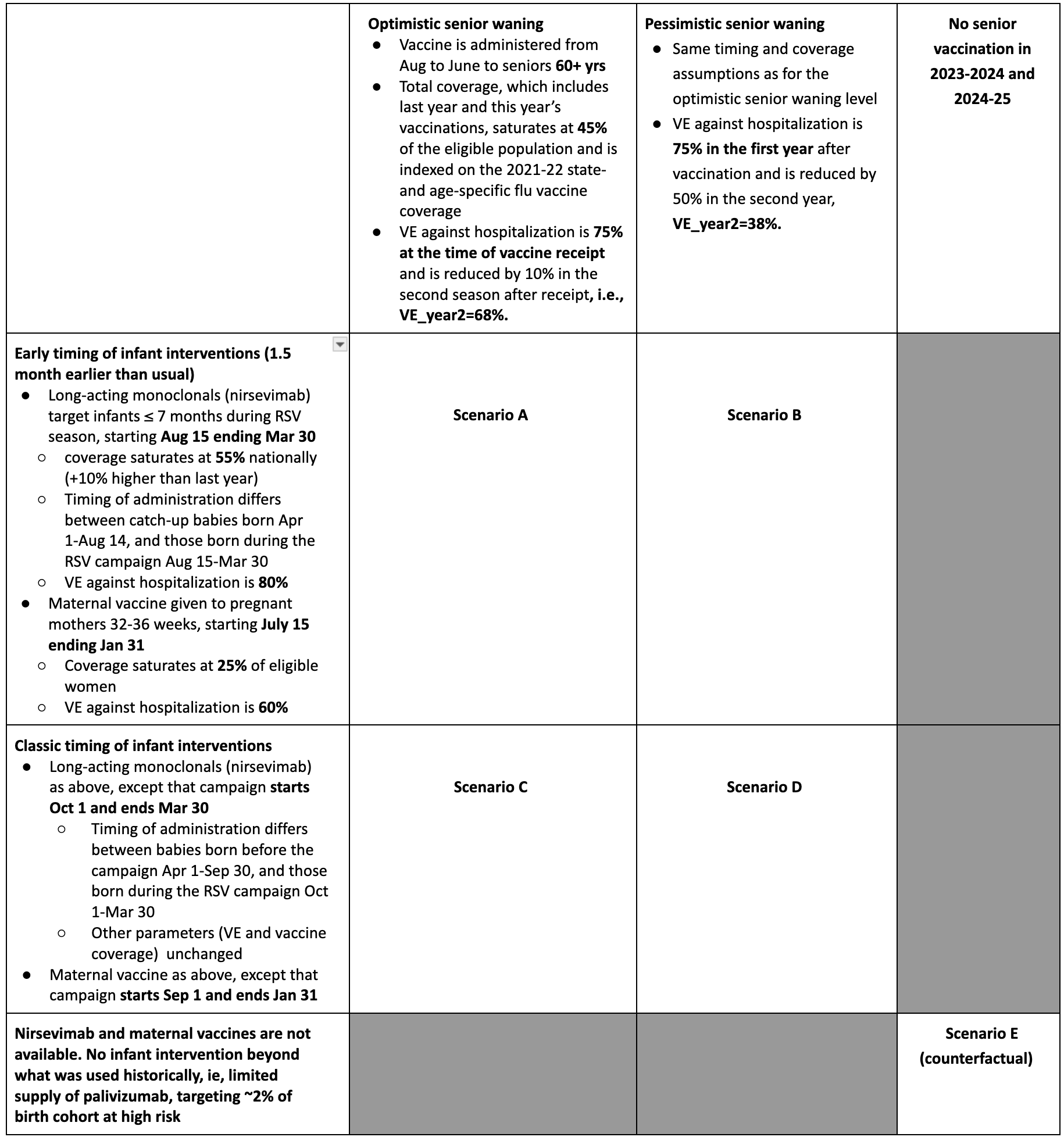
Table 1. RSV Scenario Modeling Hub round 1 2024-2025 scenarios. More detailed scenario definitions and model characteristics can be found at https://github.com/midas-network/rsv-scenario-modeling-hub.
A Note on RSV Scenario Modeling Hub Round 1 (February 26, 2024)
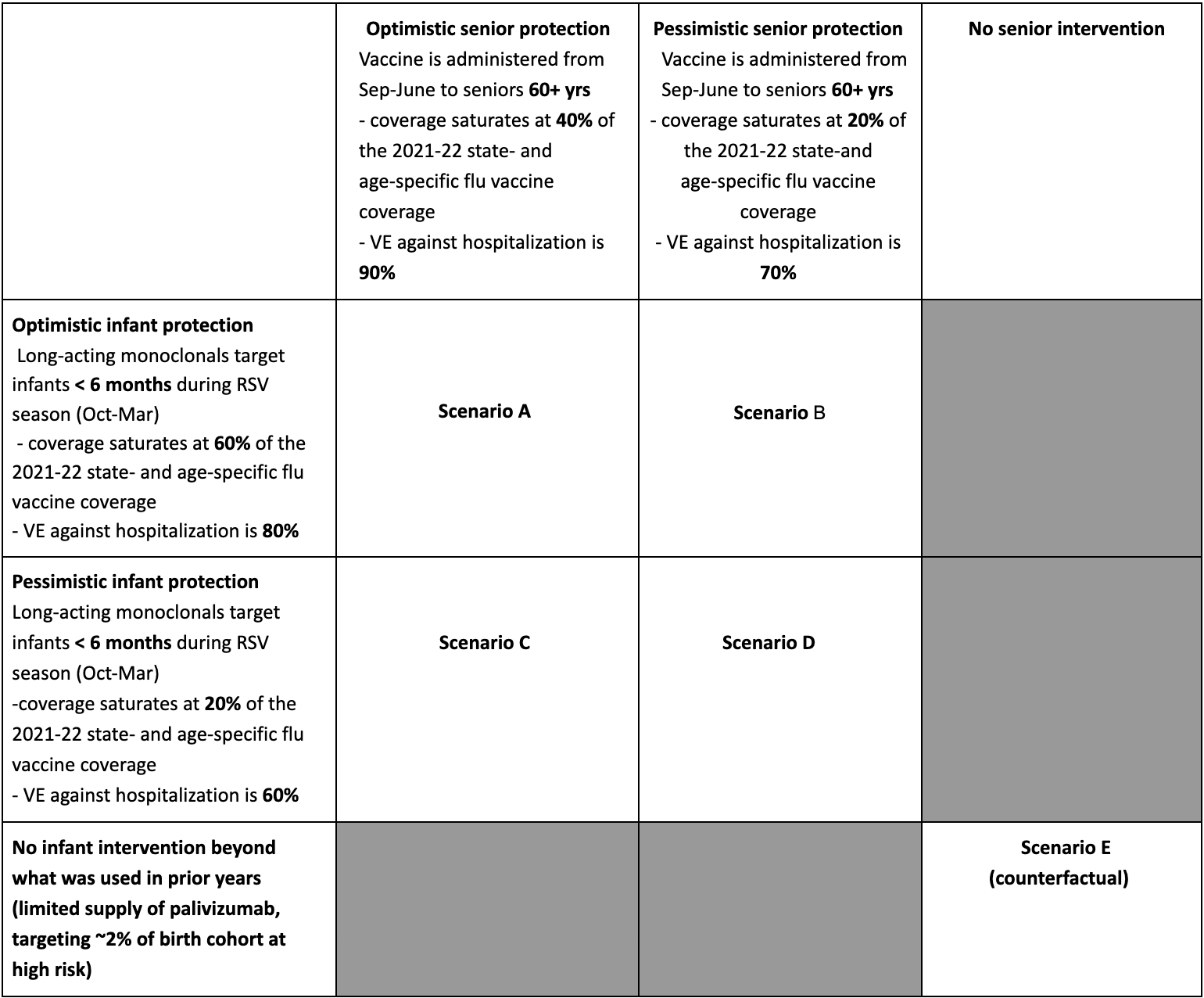
In the first round of RSV scenario projection, the RSV Scenario Modeling Hub generated mid-season hospitalization estimates for the 29-week period Nov 12, 2023 to June 1, 2024. We considered 4 intervention scenarios representing the impact of new interventions to mitigate the burden of RSV, and one counterfactual “status quo” scenario where RSV control mirrors past years. Intervention scenarios assumed optimistic and pessimistic levels of coverage and effectiveness of long-acting monoclonal antibodies (nirsevimab) in infants under 6 mo, combined with optimistic and pessimistic levels of coverage and effectiveness of vaccination in seniors 60+ yrs. Coverage was assumed to saturate at 12% (pessimistic) or 36% (optimistic) among infants, and 14% (pessimistic) or 29% (optimistic) among seniors, indexed on past coverage of influenza vaccines by state and age group. Assumptions regarding efficacy come from randomized control trials for RSV interventions. Ensemble projections are based on contributions from 10 teams, using the trimmed linear opinion pool aggregation approach. All-age and age-specific estimates of RSV hospitalizations are provided nationally and for 12 states contributing to RSV-NET surveillance.
Our main findings include:
- 11% (95% CI 6-16%) of seasonal RSV hospitalizations, or 15,500 nationally (95%CI 9,300-21,600), are projected to be averted in the most optimistic scenario versus no intervention (scenario A, optimistic intervention coverage and high effectiveness in both infants and seniors, compared to status quo scenario).
- With the most pessimistic assumptions, interventions avert 4% (95% CI 2-6%), or 5,800 (95% CI 3,200-8,400) average hospitalizations (scenario D, pessimistic intervention coverage and moderate effectiveness in both infants and seniors, compared to status quo scenario).
- Projected intervention benefits are highest in the targeted age groups: 21% (95% CI 16-24%) of RSV hospitalizations would be averted among seniors over 65 yrs and 15% (95% CI 4-24%) among infants under 1 yr, with 56% of total hospitalizations averted among infants (optimistic scenario).
- These results correspond to 1.24 (95% CI 0.45-2.03) infant hospitalization averted per 100 doses of monoclonals delivered in 2023-24) and 0.04 (0.03-0.05) senior hospitalization averted per 100 vaccine doses (optimistic scenario).
- The peak and cumulative hospitalization burden of the 2023-24 RSV season is likely to remain lower than that of the last season which had seen a large and unusual post-COVID-19 rebound of RSV, especially in children.
- Cumulative RSV hospitalizations for the period July 1, 2023 - June 1, 2024 are projected to reach 168,000 (95,500-303,000) for the most optimistic scenario compared with 183,000 (95%PI 106,000-315,000) for the counterfactual scenario.
- Ensemble projections suggest a prolonged period of RSV activity between November and March, with a peak most likely to occur in mid-December 2023. There is heterogeneity in projected RSV timing between the 12 states considered in this analysis, with most likely peak dates ranging between Nov 15, 2023 (Georgia, Tennessee) and Jan 15, 2024 (Utah).
- The combined impact of RSV, influenza, and COVID-19 on hospitalizations is likely to remain below that of last season (2022-23). Of note in 2022-23, there was concurrent activity of influenza, COVID-19, and RSV early in the season, resulting in a particularly high total hospitalization burden. Activity of these pathogens is projected to be more asynchronous this year.
- There is variability between states in the projected timing and intensity of RSV activity this season, due in part to geographic differences in the seasonality of RSV, impact of COVID-19 NPIs since 2020, and differences in testing and transmission intensities.
A few caveats are worth noting:
- Based on recent data on RSV intervention uptake in the US, our optimistic scenario A seems most closely aligned with reality.
- There are differences in the post-pandemic rebound of RSV across age groups, which are not fully understood nor accounted for by all models. In particular, projections tend to underestimate disease burden in seniors. These differences could be in part due to different strengths of NPIs between young children and adults during the COVID-19 period (e.g., masking propensities), and age-specific changes in RSV reporting.
- This is the first round of RSV projections, and there is limited availability of calibration data or past RSV modeling experience. This is the first time that the RSV-NET hospitalization dataset has been used for long-term projections. RSV-NET covers a fraction of 12 states (9% of the US population overall).
- Most models assume that RSV interventions will not reduce infection or transmission, so indirect benefits are close to zero in ensemble estimates.
- Testing practices continue to evolve in the wake of the COVID-19 pandemic (e.g., increased use of multi-pathogen testing), which may affect reported hospitalizations in the RSV-NET system. This will affect comparison with our projections and with prior year hospitalization data.
For more detailed information, please consult the RSV Round 1 Scenario Modeling Hub Report available on the RSV Scenario Modeling Hub Github.
Table 1. RSV Scenario Modeling Hub round 1 2023-2024 scenarios. More detailed scenario definitions and model characteristics can be found at https://github.com/midas-network/rsv-scenario-modeling-hub.